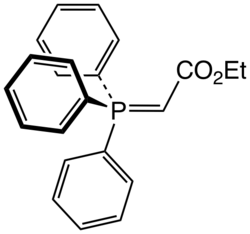
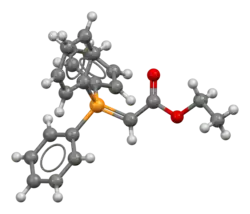
Triphenylcarbethoxymethylenephosphorane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Ethyl (triphenyl-λ5-phosphanylidene)acetate | |||
| Other names
(2-Ethoxy-2-oxoethylidene)triphenylphosphorane
(Carbethoxymethylene)triphenylphosphorane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.012.865 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C22H21O2P | |||
| Molar mass | 348.382 g·mol−1 | ||
| Melting point | 124 to 129 °C (255 to 264 °F; 397 to 402 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |||
Triphenylcarbethoxymethylenephosphorane is an organophosphorus compound with the chemical formula Ph3PCHCO2Et (Ph = phenyl, Et = ethyl). It is a white solid that is soluble in organic solvents.
The compound is a Wittig reagent. It is used to replace oxygen centres in ketones and aldehydes with CHCO2Et.[1]
References
- ^ Lang, R. W.; Hansen, H.-J. (1984). "α-Allenic Esters from α-Phosphoranylidene Esters and Acid Chlorides: Ethyl 2,3-Pentadienoate". Organic Syntheses. 62: 202
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 7, p. 232.